 

 

 

 

 
 |
AuRx is currently seeking highly-qualified investors. This may be a single investment
or a combination of many. Please contact Dr. Gary Calton at gcalton@aurx.com
for more information.
This investment will allow AuRx Inc. to build and operate a production
plant. Production and sales will coincide with the Phase 3 clinical
trial which we have been authorized to begin.
Newest Press Release: September 2006
 Skip directly to our latest
press releases by clicking here or the "Management" button.
Skip directly to our latest
press releases by clicking here or the "Management" button.
Our Clinical Trial Registry gives you a way of receiving direct information about upcoming trials, press releases and other pertinent information about the therapy. The registry also gives AuRx a database needed in our efforts to secure funding to continue the clinical trials.
The registry form is transmitted on a secure page, shown by the lock icon at the bottom of your screen and the �S� in the �https� in the web address. Access to your information is very tightly controlled and extremely limited ensuring your confidentiality.
Thank you for registering!
Sign
up for our Clinical Trial Registry!
A comparison between the best currently available treatment for genital herpes, Valtrex (data taken from package insert), and the AuRx immunotherapy.
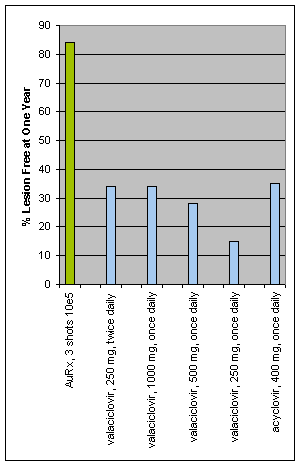 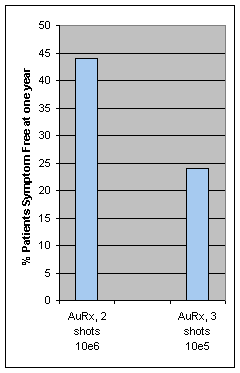
There are a number of differences in the patients enrolled in the trials.
1. For valaciclovir, recurrences were scored only for patients having lesions, whereas for AuRx, recurrences were scored on the basis of any sign or symptom.
2. For valaciclovir, the patients enrolled had a median of 5 outbreaks per year, whereas the patients in the AuRx study had an average of 9 outbreaks, with a minimum of 5 per year. The valaciclovir study results were 25% less effective for patients having more than 10 recurrences per year.
3. For valaciclovir, the percentage of patients having a placebo effect was much lower than for the AuRx study.
4. The dose of the AuRx immunotherapy has not been optimized, whereas the valaciclovir studies were done over a period more than ten years and designed for optimizing the dose and treatment schedule.
Reitano M, Tyring S, Lang W, Thoming C, Worm AM, Borelli S, Chambers LO, Robinson JM, Corey L. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. International Valaciclovir HSV Study Group. J Infect Dis. 1998 Sep;178(3):603-10
A randomized double-blind trial to evaluate the safety of a novel
recombinant virus, ICP10deltaPK, for reduction or prevention of
recurrent herpes simplex virus type 2 (HSV-2) infection was carried
out in public hospitals in Mexico City. Persons having a minimum
of 5 documented herpetic recurrences in the previous year were randomized
for vaccination. Patients were examined within 72 hours of lesion
occurrence. If accepted into the study, the patient was inoculated
subcutaneously in the upper deltoid muscle area at days 7, 17, and
28 after initiation of lesion occurrence. Recurrences were recorded
by patient diary and physician examination. During the observation
period (extending from 10 to 180 days after the last booster dose),
recurrences in the vaccine (V) group were prevented completely in
37.5% of the patients, whereas in the placebo (P) group, 100% of
the patients had at least one recurrence (P = .068). Vaccinated
patients had fewer recurrences (V, 1.58; P, 3.13 [P = .028]). The
mean number of illness days was 10 for the vaccine group and 18
for the placebo group (P = .028).
Casanova
G, Cancela R, Alonzo L, Benuto R, del Carmen Magana M, Hurley DP,
Fishbein E, Lara C, Gonzalez T, Ponce R, Burnett JW, Calton GJ.
A double-blind study of the efficacy and safety of the ICP10DPK
vaccine against recurrent genital HSV-2 infections. Cutis. 2002;70(4):235-239
continue
|